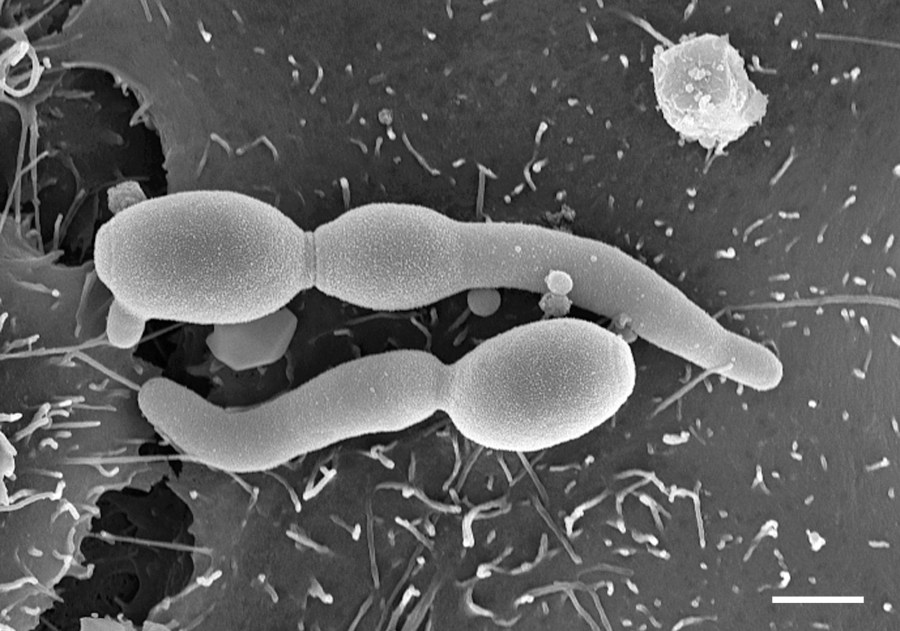
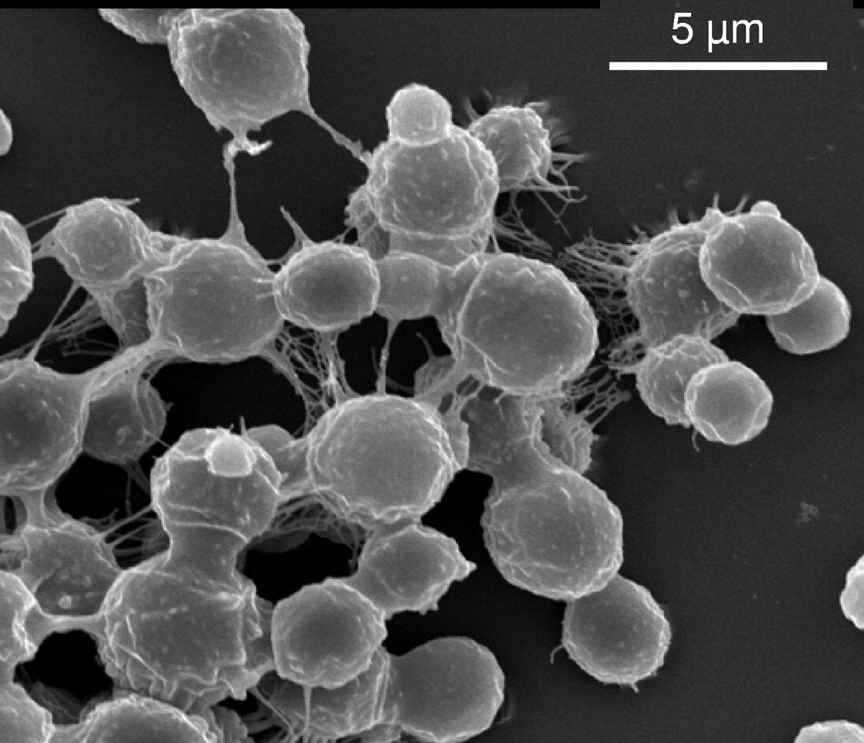
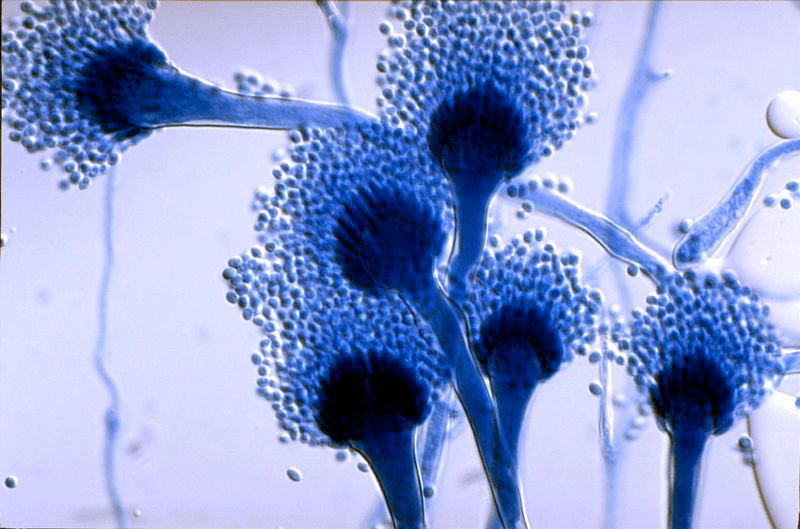
Human pathogens
Elaine Bignell is Professor of Medical Mycology at the MRC Centre for Medical Mycology at the University of Exeter, where she is also Co-Director of Research. A fungal geneticist by training, Elaine’s research seeks a mechanistic understanding of fungal lung disease with a view to developing novel diagnostics and antifungal therapies.
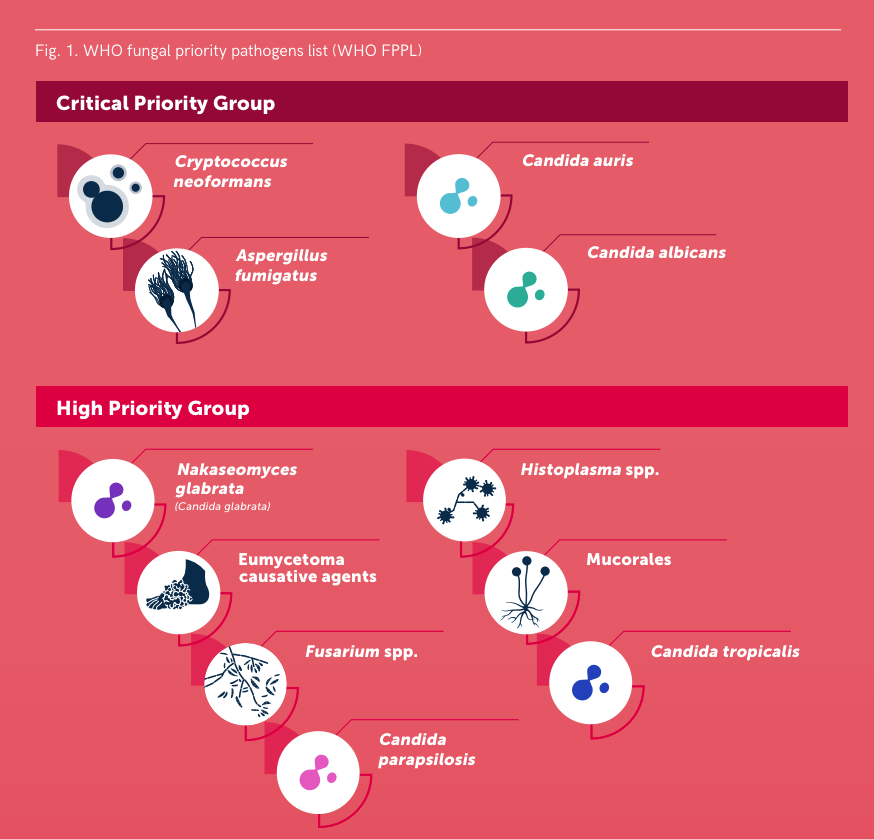
Diseases caused by fungi kill more than 1.5 million people per year and lessen quality of life in millions more. Such diseases are difficult to diagnose and treat, and the underlying pathobiology is poorly understood. For many decades this problem has been neglected by funders and policy makers however, due to significant threats posed by antifungal drug resistance, epidemic zoonoses, and the rapid emergence of a new multidrug resistant fungal species, the World Health Organisation (WHO) recently issued a first WHO priority fungal pathogens list. Focusing on fungal pathogens that can cause invasive acute and subacute systemic fungal infections, and whose treatment is challenging and/or threatened by antifungal drug resistance, pathogens were ranked and categorised into critical, high or moderate risk groups. The report is an excellent source of information on the priority pathogens and diseases they cause. Important to note that this is not an exhaustive list of important human pathogens, all of which required focused attention to strengthen our knowledge of human diseases.
Antifungal drug resistance
Clinical antifungal therapy is reliant upon a small number of drug classes, resistance to which is growing year on and year and is fuelled by prolonged exposure to, or suboptimal use of clinical antifungal drugs, as well as fungicide use in urban, industrial and agricultural settings. For an excellent review see Tackling the emerging threat of antifungal resistance to human health. The mechanistic basis of antifungal drug resistance is an important research field that is rapidly advancing, revealing the importance of genetic, epigenetic and physiological drivers of drug resistance as well as highlighting the astonishing, yet somewhat worrisome versatility of fungi when placed under selective pressure. As well as acquired drug resistance, some pathogens (including the newly emerged Candida auris)harbour intrinsic multidrug resistance Candida auris: an emerging antimicrobial-resistant organism with the highest level of concern and some fungal pathogens, such as Mucorales species, are resistant to almost all available antifungal drugs. Despite the progression of several novel agents through the antifungal development pipeline The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin, continued reliance upon monotherapy and dual use of drug classes in the clinic, and industrial and/or agricultural settings will continue to exert positive selection for clinical antifungal resistance.
Phenotypic plasticity
The ability to adapt rapidly to extracellular stress is a common to all fungi. A multiplicity of receptors, signalling pathways and regulatory proteins have been identified and their functions characterised in exquisite detail, providing a rich hunting ground for novel antifungal therapies. There is growing appreciation for the relevance of genetic and epigenetic plasticity as a driving force for stress adaptation and tolerance, including to antifungal drug exposure. For example, transposon mobilisation, aneuploidy and chromatin structure, hypermutation and RNAi have all been recently implicated in promoting stress adaptation in human fungal pathogens, for examples read: Transposon mobilization in the human fungal pathogen Cryptococcus is mutagenic during infection and promotes drug resistance in vitro; Aneuploidy and isochromosome formation in drug-resistant Candida albicans ; Chromatin Structure and Drug Resistance in Candida spp. ; Heterochromatin and RNAi act independently to ensure genome stability in Mucorales human fungal pathogens.
Virulence factors
Compared to understanding of host pathogen interactions in fungal diseases of plants, the modus operandi of most human fungal pathogens remains poorly understood. In part this is due to the relative infancy of the field (human diseases have increased in prevalence only over the last 40 years) and to the complexity of studying disease processes in whole animals. Undoubtedly, more capacity is needed in the field of medical mycology, and harnessing knowledge from across the wider mycology discipline will be an important onward strategy. Major human pathogens are either commensal organisms (like most Candida species) or are prevalent in the natural environment (like Aspergillus, Cryptococcus, Fusarium and Mucorales species). Whether or not human fungal pathogens rely upon a wealth of virulence proteins (as well documented for phytopathogenic fungi) remains to be seen. Certainly, there have been multiple new examples of fungal proteins (multiple types, differentially present in various human fungal pathogens) that subvert human defences and exert dominant negative effects upon disease outcomes in whole animals. For examples read: Candidalysins Are a New Family of Cytolytic Fungal Peptide Toxins ; Aspergillus fumigatus hijacks human p11 to redirect fungal-containing phagosomes to non-degradative pathway; Secreted fungal virulence effector triggers allergic inflammation via TLR4
Future horizons and fungal threats
As we advance into a future threatened by climate change and drug resistance, it is important that we acquire the knowledge and know-how to understand and manage fungi that are, or could become, life-threatening pathogens. Surveillance, underpinned by genome data, will be a major vehicle for detecting and understanding shifts in epidemiology as will metagenomic analyses of the biomes of susceptible hosts and multiple other niches Fungal Diseases in the 21st Century: The Near and Far Horizons – PMC (nih.gov).